Would you like to take part in a clinical study for a new investigational treatment for Prurigo Nodularis at no cost to you?
No health insurance is required to participate. You will receive all study related care from a dedicated study team at no cost. The study will include visits to a clinic in your location.
You may qualify for a clinical study if you:
- Are over the age of 18
- Have Prurigo Nodularis
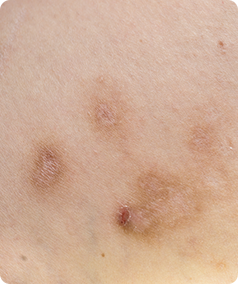
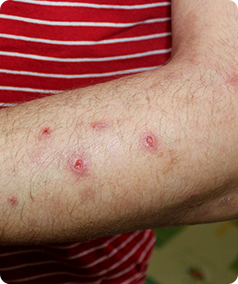
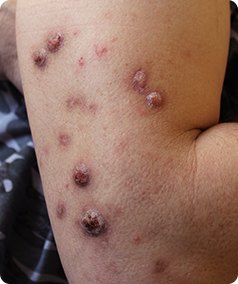
- Have approximately 20 nodules on your body
Sign Up
If you think you might like to join this study or would like more information, please enter your information below so we can see if you qualify and can contact you about the study. Remember, participation is entirely voluntary. Even if you decide to take part, it is absolutely fine if you change your mind later.
About Prurigo Nodularis
Prurigo Nodularis is a rare skin condition that causes the formation of hard, itchy lumps referred to as ‘nodules’ on the skin. The itching can be very intense, which can cause scratching that leads to pain and bleeding. This itching is worsened by heat, sweating and irritants such as clothing rubbing on the affected area.
Although scratching is known to cause more nodules to appear, the exact cause is unknown as to why the intense itching and nodules appear in the first place. It is not thought that PN is inherited, however, because conditions such as eczema may contribute to the condition, some instances of PN may be hereditary, which is related to family history and environmental factors, such as the climate which someone lives in.
A diagnosis of Prurigo Nodularis is typically done by a skin biopsy, where it can be seen under a microscope that the nerves in the skin are thickened because of the condition. After a diagnosis, further testing may be performed such as blood tests, to ensure there are no other issues related to liver or kidney function.
Although treatments may help relieve the symptoms of Prurigo Nodularis, there is no cure. Topical steroid creams, oral corticosteroids and antihistamines can be effective for some cases in managing the symptoms, as well as habit-reversal therapy that can help assist with resisting the urge to scratch.
Frequently Asked Questions
What is a clinical trial or research study?
A clinical trial, also referred to as a research study, is a scientific study that evaluates the safety and efficacy of an investigational medication. A research study may show that the investigational medication is better than, as good as, or worse than the standard treatment or inactive placebo. Qualified doctors, nurses and other medical professionals will conduct the study.
It is only through the completion of research studies that investigational medication can be evaluated, and if proven safe and effective, approved for general use by appropriate regulatory or health authorities, such as the U.S. Food and Drug Administration (FDA). Prescription medications in use today were first proven safe and effective in research studies.
Is there compensation for time and travel?
Participants who are eligible and take part in the study may receive compensation for time and travel. Please discuss this with the research site staff when they contact you.
Can participation in the study stop at any time?
A participant can stop participating in the study at any time. If you do decide to stop early, you will simply have to notify the study team at the research center. The participant may be asked to visit one last time to check up on their health once the investigational medication has been stopped.
How far is the study clinic?
We match you to a study clinic within a close travel distance from your home. If we are not running the study in your area currently, with your permission, we will keep you in our database and reach out once a study clinic in your area becomes available. If, at any time, you decide you no longer want to participate in the study, you can opt out and we will delete your information.
What happens to personal information?
When you sign up for a research study, your personal information is protected as required by law. The research team stores personal and private information with codes (instead of names or other identifying information), in order to not identify the participant or volunteer. The informed consent form that will be provided to you by the research team will have more information about privacy protection.
